Neuron Modeling¶
Neurons are modelled as equivalent electrical circuits. The morphology of a neuron can be broken into isopotential compartments connected by axial resistances Ra denoting the cytoplasmic resistance. In each compartment, the neuronal membrane is represented as a capacitance Cm with a shunt leak resistance Rm. Electrochemical gradient (due to ion pumps) across the leaky membrane causes a voltage drive Em, that hyperpolarizes the inside of the cell membrane compared to the outside.
Each voltage dependent ion channel, present on the membrane, is modelled as a voltage dependent conductance Gk with gating kinetics, in series with an electrochemical voltage drive (battery) Ek, across the membrane capacitance Cm, as in the figure below.
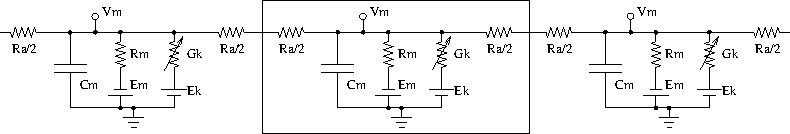
Equivalent circuit of neuronal compartments
Neurons fire action potentials / spikes (sharp rise and fall of membrane potential Vm) due to voltage dependent channels. These result in opening of excitatory / inhibitory synaptic channels (conductances with batteries, similar to voltage gated channels) on other connected neurons in the network.
MOOSE can handle large networks of detailed neurons, each with complicated channel dynamics. Further, MOOSE can integrate chemical signalling with electrical activity. Presently, creating and simulating these requires PyMOOSE scripting, but these will be incorporated into the GUI in the future.
To understand channel kinetics and neuronal action potentials, run the Squid Axon demo installed along with MOOSEGUI and consult its help/tutorial.
Read more about compartmental modelling in the first few chapters of the Book of Genesis.
Models can be defined in NeuroML, an XML format which is mostly supported across simulators. Channels, neuronal morphology (compartments), and networks can be specified using various levels of NeuroML, namely ChannelML, MorphML and NetworkML. Importing of cell models in the GENESIS .p format is supported for backward compatibitility.
Modeling details¶
Some salient properties of neuronal building blocks in MOOSE are described below. Variables that are updated at every simulation time step are listed dynamically. Rest are parameters.
Compartment When you select a compartment, you can view and edit its properties in the right pane. Vm and Imare plot-able.
- Vm
- membrane potential (across Cm) in Volts. It is a dynamical variable.
- Cm
- membrane capacitance in Farads.
- Em
- membrane leak potential in Volts due to the electrochemical gradient setup by ion pumps.
- Im
- current in Amperes across the membrane via leak resistance Rm.
- inject
- current in Amperes injected externally into the compartment.
- initVm
- initial Vm in Volts.
- Rm
- membrane leak resistance in Ohms due to leaky channels.
- diameter
- diameter of the compartment in metres.
- length
- length of the compartment in metres.
HHChannel Hodgkin-Huxley channel with voltage dependent dynamical gates.
- Gbar
peak channel conductance in Siemens.
- Ek
reversal potential of the channel, due to electrochemical gradient of the ion(s) it allows.
- Gk
conductance of the channel in Siemens. Gk(t) = Gbar × X(t)Xpower × Y(t)Ypower × Z(t)Zpower
- Ik
- current through the channel into the neuron in Amperes.
Ik(t) = Gk(t) × (Ek-Vm(t))
- X, Y, Z
gating variables (range 0.0 to 1.0) that may turn on or off as voltage increases with different time constants.
dX(t)/dt = Xinf/τ - X(t)/τ
Here, Xinf and τ are typically sigmoidal/linear/linear-sigmoidal functions of membrane potential Vm, which are described in a ChannelML file and presently not editable from MOOSEGUI. Thus, a gate may open (Xinf(Vm) → 1) or close (Xinf(Vm) → 0) on increasing Vm, with time constant τ(Vm).
- Xpower, Ypower, Zpower
powers to which gates are raised in the Gk(t) formula above.
HHChannel2D The Hodgkin-Huxley channel2D can have the usual voltage dependent dynamical gates, and also gates that depend on voltage and an ionic concentration, as for say Ca-dependent K conductance. It has the properties of HHChannel above, and a few more, similar to in the GENESIS tab2Dchannel reference.
CaConc This is a pool of Ca ions in each compartment, in a shell volume under the cell membrane. The dynamical Ca concentration increases when Ca channels open, and decays back to resting with a specified time constant τ. Its concentration controls Ca-dependent K channels, etc.
- Ca
Ca concentration in the pool in units mM ( i.e., mol/m3).
d[Ca2+]/dt = B × ICa - [Ca2+]/τ
- CaBasal/Ca_base
Base Ca concentration to which the Ca decays
- tau
time constant with which the Ca concentration decays to the base Ca level.
- B
constant in the [Ca2+] equation above.
- thick
thickness of the Ca shell within the cell membrane which is used to calculate B (see Chapter 19 of Book of GENESIS.)